Understanding Sunscreens
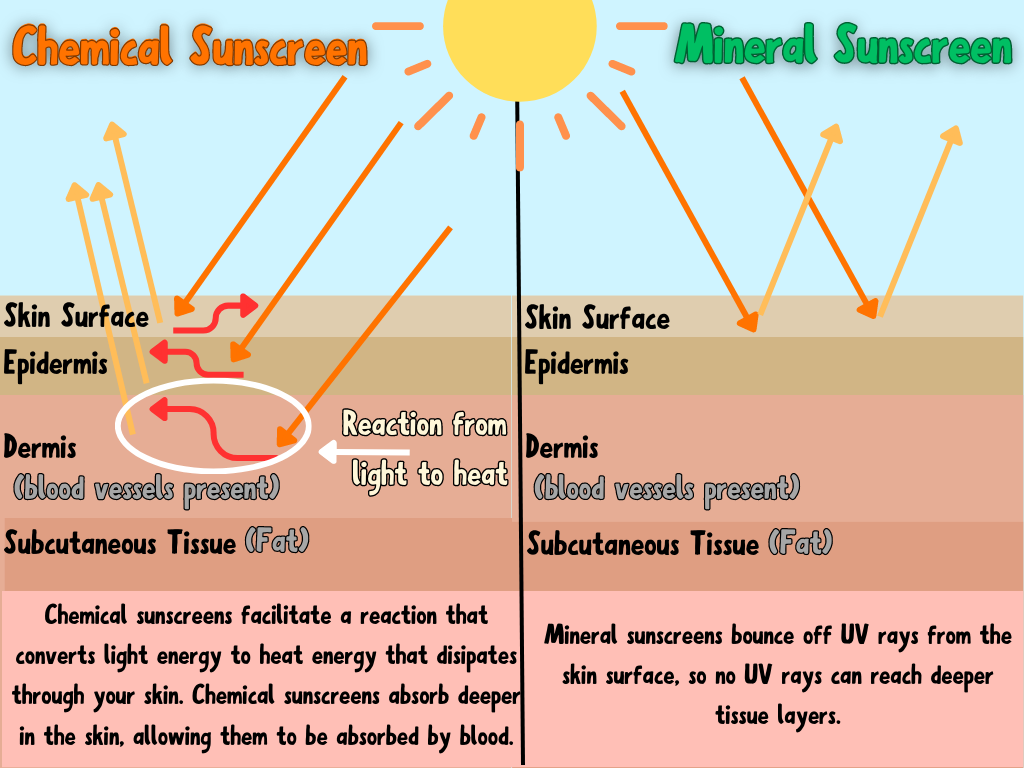
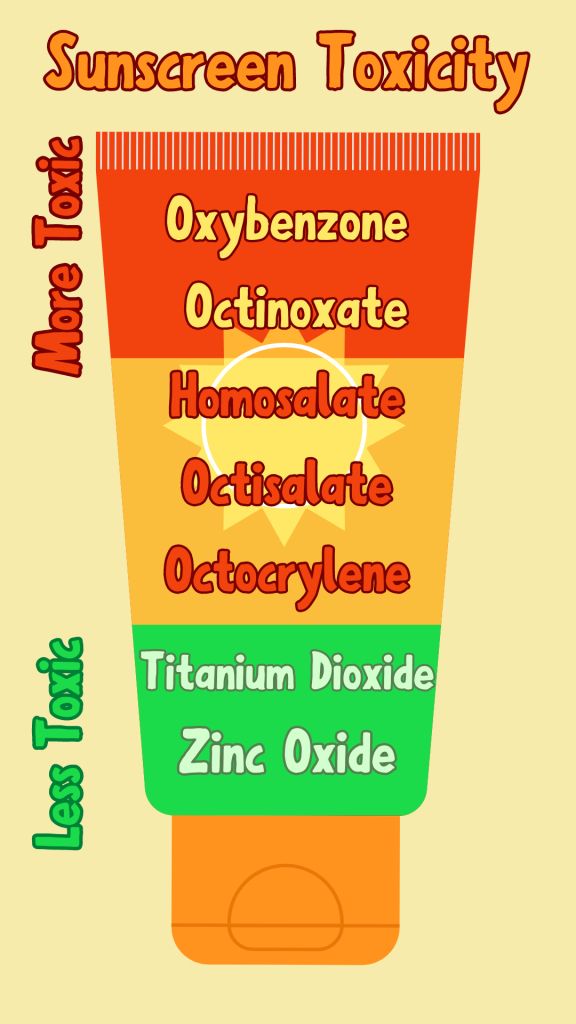
Look for Titanium Dioxide or Zinc Oxide as the active ingredients in your sunscreen.
AVOID chemicals like Oxybenzone and Octinoxate.
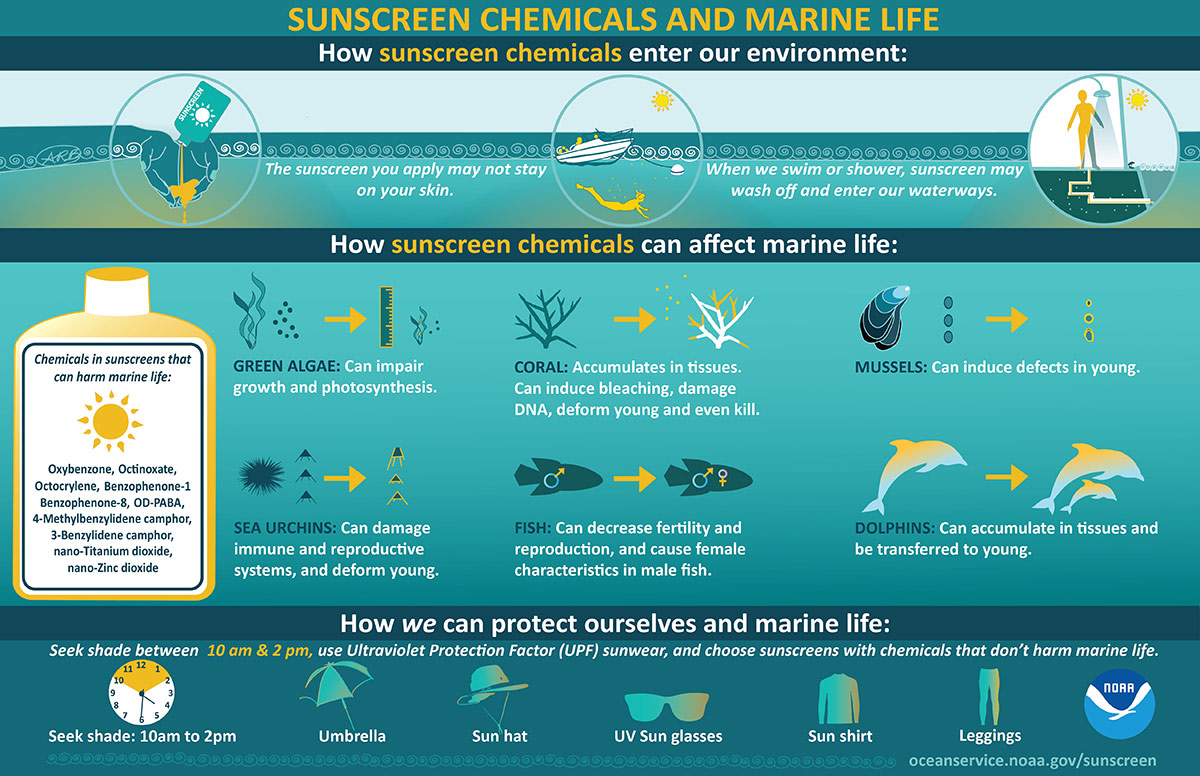
Sunscreen is something we all wear, or are hopefully all wearing, almost every day. Whether it’s in your face moisturizer or you’re lathering up in SPF each morning, sunscreen is one of our essential toiletries. We all rely on sunscreen to protect our skin, but what if the thing that’s supposed to protect us hurts us or our environment?
Unfortunately, that is happening due to some of the chemicals in our sunscreens. Chemicals like Oxybenzone and Octinoxate have been proven to cause harm to us, as well as our environment. When swimmers get in the water wearing sunscreens with these chemicals, they are directly contaminating that water source. The residual sunscreen that hasn’t been absorbed into the skin washes into the water and is usually kicked toward the reefs through swimming actions.
Oxybenzone is found in over 3500 sunscreen products worldwide, making it very common in sunscreens. Oxybenzone is found in many aquatic environments and has been shown to disrupt coral reproduction, cause coral bleaching, and damage coral DNA. When absorbed through the skin, Oxybenzone can be found in urine as quickly as 30 minutes after application, allowing it to bioaccumulate in our bodies. Bioaccumulation is the process of a build-up of certain toxins or chemicals in our bodies that we were exposed to over time; the little bit left behind in our system accumulates until there is a large amount. Oxybenzone has been found in bird eggs, fish, coral, humans, and other marine animals. Oxybenzone can be biomagnified if we eat a fish with high concentrations of oxybenzone, giving us a large amount of that toxic chemical in our body. This is biomagnification, where one predator animal ingests a prey organism with a high concentration of some toxin build up in its body, passing the toxins onto the predator (which could be human) and consuming that prey. Essentially, biomagnification means chemicals like Oxybenzone may increase in concentration in the tissues of organisms as it travels up the food chain. The more Oxybenzone washed into the water, the more likely it is consumed by a smaller organism which is then consumed by a human who has ingested large amounts of a toxin. The abundance of Oxybenzone in our waters makes this more likely; it is found in parts per trillion concentrations off the coast of Barrow, Alaska, U.S.A, to parts per billion on coral reefs in the Caribbean, Pacific, and Red Sea. One of the highest concentrations measured in the marine environment was in Trunk Bay in the U.S. Virgin Islands National Park, St. John Island, U.S. Virgin Islands, where the beach can have up to 5,000 people daily.
Octinoxate is absorbed directly through the skin by applying sunscreens and other personal care products. It has been shown to absorb less into the skin, leaving more to wash into the water. Octinoxate can potentially be stored in the body long-term as fat tissue or in lipid-rich tissue due to it being a fat-soluble chemical. Octinoxate is also at risk of being bioaccumulated in organisms and biomagnified through the food web. A number of aquatic and marine species have been discovered to be contaminated, from carp, catfish, eel, white fish, trout, barb, chub, perch, and mussels to coral, mahi-mahi, dolphins, sea turtle eggs, and migratory bird egg. Octinoxate is also found in large concentrations; in coral reef environments, Octinoxate can reach more than 10 parts per billion. Along the west coast of Maui in 2015, in Hawaii, 11 coral reef sites that were sampled had Octinoxate concentrations from 6.9 parts per trillion to 1,516 parts per trillion.
Some brands we love :
- All Good: All Sunscreen Lotions and Sticks
- Badger: All Sunscreen Lotions and Sticks
- Goddess Garden Organics: All Sunscreen Lotions, Sticks, and Lip Balms
- Kōkua Sun Care: Natural Zinc Sunscreen
- MyChelle Dermaceuticals: All Sunscreen Lotions, Sticks, and Liquids
- Raw Elements: All sunscreen products
- Raw Love: All Natural Mineral Sunscreen
- Stream2Sea: All sunscreen products
- TreeActiv: Safflower Oleosomes Daily Natural Moisturizing Face Sunscreen SPF 30